Bioactive Glasses
Bioactive glass particles (left) and apatite crystals (right) occluding dentinal tubules
Image: Delia BrauerBioactive glasses surface react with physiological fluids and form apatite on their surface. They are used clinically as bone grafts as they form an intimate bond with living bone and, over time, fully degrade in the body. Recently, they also have attracted interest for use in oral care materials, such as toothpaste for treating dentine hypersensitivity and aiding tooth remineralisation. Bioactive glasses act as release vehicles for therapeutically active ions such as fluoride (increases bone formation and prevents dental caries) or zinc (bactericidal).
Phosphate Glasses
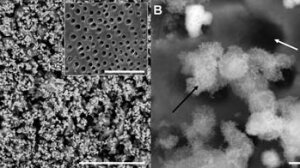
Porous sintered phosphate glass scaffolds
Image: Delia BrauerPhosphate glasses, as the name suggests, consist of phosphate (rather than silicate or borosilicate) as the glass former. They may dissolve completely in aqueous solution, and their solubility can easily be adjusted via their composition. Therapeutic ions such as strontium, zinc or fluoride can be incorporated into the glass to be released upon dissolution, for stimulation of bone growth, wound healing or for prevention of infections. We are particularly interested in the mechanism of phosphate glass dissolution, which we study using various techniques including 31P NMR spectroscopy.
Aluminosilicate Glasses
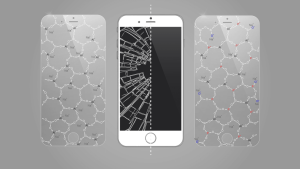
Aluminosilicate glass as a smartphone display cover
Picture: Paul WiemuthAluminosilicate glasses are highly relevant for engineering applications such as glass fibres for use in composites (e.g. for wind turbine blades) or as touchscreen covers. As glass-ceramics, they are used for example as cooker tops. We are interested to see how changes in glass composition and structure affect glass properties such as chemical durability, mechanical or thermal properties.
In the biomedical area, aluminosilcate glasses are used in glass ionomer dental cements (see below).
Glass Ionomer Cements
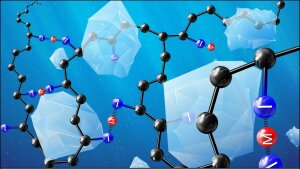
Schematic glass ionomer cement
Picture: Paul WiemuthGlass ionomer cements are commonly used in dentistry. As they form a chemical bond to bone, they are also of potential interest for orthopaedic applications. Glass ionomer cements set by an acid-base reaction between an acid-degradable glass and a polymeric acid such as poly(acrylic acid). Dental ionomer cements contain aluminium, which plays an important role in cement setting and stability. However, as aluminium is a neurotoxin and also negatively affects bone mineralisation, we are interested in new aluminium-free ionomer cements as an alternative for orthopaedic applications.
Composites
Glasses are commonly known to be brittle, and fabrication of glass fibres is a way to produce a glassy material with improved mechanical properties. The good mechanical properties of glass fibres make degradable phosphate glass fibres of interest in the field of biomaterials, for example, fabrication of bone fracture fixation materials such as glass-fibre-reinforced degradable polymers, but also as meshes or woven fibre constructs for tissue engineering or soft tissue replacement. Degradable fracture fixation materials offer the advantage that they can degrade in the body, obviating the need for a second operation to remove hardware.
Glass Structure
We are interested in the interplay between glass composition, structure and properties. A thorough understanding of how the atomic set-up determines glass properties allows for the precise tailoring of glass properties to meet the requirements of specific applications. Owing to the amorphous structure of glasses, structural analyses remain challenging and typically require a combination of complementary methods including solid-state nuclear magnetic resonance (NMR) and vibrational (infrared and Raman) spectroscopy.